
Compensation
Up to $2000
For Completed Study
Psoriatic Arthritis Clinical research study
Diagnosed With Psoriatic Arthritis?
Are you living with Psoriatic Arthritis and interested in advancing medical research? Participate in our clinical trial to help explore new treatment options while receiving compensation for your involvement.
Discover if you qualify, learn more about the trial process, and see how your participation can contribute to the future of Psoriatic Arthritis treatments. Sign up below to begin!
What is Psoriatic Arthritis?
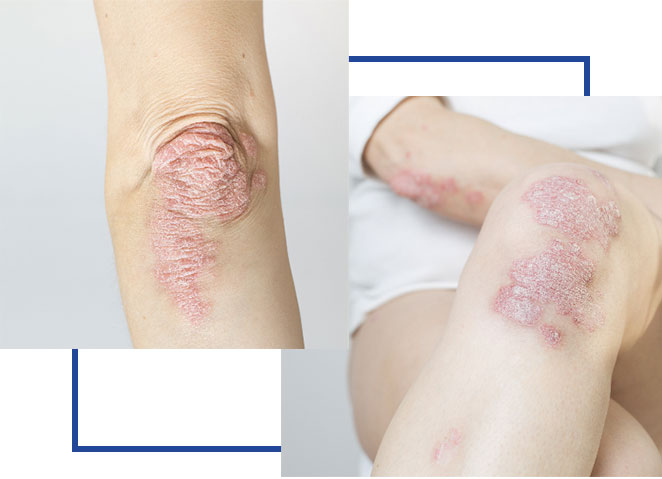
Psoriatic Arthritis (PsA) is a long-term autoimmune disease that causes swelling, pain, and stiffness in the joints. PsA happens because the immune system mistakenly attacks healthy joints and is treated by a Rheumatologist or Dermatologist. It can affect any joint but most often targets the fingers, toes, lower back, knees, and ankles. It may also cause the fingers or toes to swell up like sausages. PsA often occurs in people who have Psoriasis, a skin condition that causes red, scaly patches and are usually the first symptoms patients notice. PsA is different from Osteoarthritis (OA), which is treated by an Orthopedic Doctor. Osteoarthritis (OA) is a “wear and tear” type of arthritis that develops as people get older or after joint injuries.
TWo Convenient pinellas county locations
Study Locations
2147 NE Coachman Road
Clearwater, FL 33765
727-466-0078
605 N. Howard Ave.
Tampa, FL 33606
813-870-1292
Indication: Psoriatic Arthritis
What To Know About Volunteering For Our Psoriatic Arthritis Clinical Trial
Joining a clinical trial is entirely voluntary, and you have the freedom to withdraw at any time. The process begins by finding a suitable trial, consulting with study doctors to determine eligibility, signing an informed consent form, completing necessary screening tests, and enrolling if you meet the requirements.
want to learn more?
What is Psoriatic Arthritis (PsA)?
Psoriatic Arthritis (PsA) is a chronic autoimmune condition that affects both the skin and joints. It occurs when the immune system mistakenly attacks healthy cells and tissues, leading to inflammation in the joints and skin. PsA is often associated with psoriasis, a skin condition that causes red, scaly patches, but it can also occur in individuals without any noticeable skin symptoms.
- PsA primarily affects the joints, causing symptoms such as:
- Joint pain, stiffness, and swelling
- Skin rashes and patches (psoriasis)
- Nail changes, including pitting or separation from the nail bed
- Swelling in fingers and toes (dactylitis)
- Reduced range of motion in affected joints
- Fatigue and general malaise
While there is no cure for PsA, there are effective treatments available to manage symptoms, reduce inflammation, and prevent further joint damage. These treatments often include medications to suppress the immune system and control skin symptoms, along with lifestyle modifications to support overall health.
Interested In Learning More






